|
H1N1 - The Pandemic of 2009: A Debrief on Prevention and Preparedness
January 10-16, 2010 marks this season’s National Influenza Vaccination Week
by Christina J. Ansted, MPH
Most of us are familiar with the flu, a 24-48 hour period of feeling miserable with bouts of nausea, fever, and general malaise. Unless you are in a high-risk population, such as infants or small children, pregnant women, people with compromised immune systems, healthcare workers, or the elderly, the flu isn’t such a big deal. But in 1918 influenza was a VERY big deal. From 1918-1919, the world, from Europe to the United States, was gripped by an influenza pandemic, which took a serious toll on the global population. During the 1920s, researchers estimated that 21.5 million people died as a result of the 1918-1919 pandemic; more recent estimates have been as high as 50 million, with an estimated 675,000 Americans among the dead. In fact, more Americans died from influenza during this period than in World War I.(1)

Photo Credit: Office of the Public Health Service Historian
Today we know the influenza virus causes the flu. But at that time physicians believed that bacteria caused influenza. Policies for hand washing and sterilization were non-existent, or rudimentary at best. But although no one understood what caused influenza, they did know that influenza could be spread through coughing, sneezing, and contact with bodily fluids such as nasal drip. Quarantines were instituted and masks were made mandatory, but they did little to stop the spread of the disease. What they needed was a vaccine, and the solution wouldn’t come for another 14 years. It was not until 1933 that the influenza A virus, which causes almost every type of endemic and pandemic influenza, was isolated. Seven years later, in 1940, the influenza B virus was isolated, followed by isolation of influenza C virus in 1950. Influenza vaccine was first introduced as a licensed product in the United States in 1944.(1)
Because of the rapid rate of mutation of the influenza virus, the effectiveness of a given vaccine usually lasts for only a year or two.(1) This rapid mutation is the primary reason why vaccines must periodically be manufactured in anticipation of upcoming flu seasons. Manufacturers work with government agencies to estimate the number of vaccines that will be needed and the vaccines are then made, but this not a foolproof system. Viruses mutate. New strains are found. Worldwide travel is common, and infectious disease experts are continually faced with the challenges of dealing with new strains.
In recent years, the United States has been faced with shortages of flu vaccine. The current vaccine shortage demonstrates the challenges inherent in ensuring an adequate and timely flu vaccine supply. Only three manufacturers produce flu vaccine for the U.S. market, and the potential is still present for future manufacturing problems such as those experienced both this year and, to a lesser degree, in previous years. When shortages occur, their effect can be exacerbated by the existing distribution system.(2) The U.S. Government holds millions of treatment courses of antiviral medications, its Strategic National Stockpile (SNS), but there are no published criteria for sequential release of these medications to States and Territories during the course of a worldwide influenza pandemic, such as the one currently caused by the pandemic influenza A (H1N1) virus.(3)
Each year in the United States, 5% to 20% of the population get the flu; on average, more than 200,000 people are hospitalized from flu-related complications and about 36,000 people die from flu-related causes annually.(4) In April 2009, a novel pandemic H1N1 influenza virus appeared, but it has not exhibited unusually high pathogenicity. Nevertheless, because this virus spreads globally, some scientists predict that mutations will increase its lethality.(5)
H1N1 or “swine flu” is an important zoonotic disease that has been recognized as an important global health problem caused by any one of several types of swine influenza virus or swine-origin influenza virus. Swine influenza virus is common throughout pig populations worldwide. Transmission of the virus from pigs to humans is not common and does not always lead to human influenza, often resulting only in the production of antibodies in the blood. Due to variability of clinical features and limited availability of laboratory facilities, the disease remains largely under-reported.(6) This virus was originally referred to as “swine flu” because laboratory testing showed that many of the genes in this new virus were very similar to influenza viruses that normally occur in pigs (swine) in North America. But further study has shown that this new virus is very different from what normally circulates in North American pigs. It has two genes from flu viruses that normally circulate in pigs in Europe and Asia and bird (avian) genes and human genes. Scientists call this a "quadruple reassortant" virus.(7)
Investigators from the Centers for Disease Control and Prevention and 25 other public health agencies around the world sought to determine the genetic origin and the antigenic characteristics of the 2009 A (H1N1) influenza virus. Specifically, they used a genetic-similarity study to determine which influenza strains previously identified had combined to produce the new A (H1N1) flu virus.(8) This study found that the 2009 A (H1N1) flu was derived from the combination of 4 separate strains of influenza, each circulating in swine populations, including an A (H1N1) strain that has been circulating in swine populations since around the time of the Spanish flu outbreak of 1918.(8) This current virus is a novel influenza A virus, more properly termed a new subtype of influenza A (H1N1) that was not previously detected in swine or humans. More important is that this new strain appears to be spread by human-to-human transmission.(9)
So what do we do? How do we protect ourselves? And how do we properly recognize and treat the infection when we see it? Common with H1N1 or swine flu are nasal congestion, sore throat, cough, and fever. Typical symptoms of influenza such as fatigue and aches and pains may also be present. Less common, however, are diarrhea or vomiting. How to be sure? The CDC currently recommends "real-time” PCR or RT-PCR for influenza A, B, H1, and H3 conducted at a State Health Department Laboratory. Currently, swine influenza A (H1N1) virus will test positive for influenza A and negative for H1 and H3 by RT-PCR. If reactivity of RT-PCR for influenza A is strong (e.g., Ct ≤ 30) it is more suggestive of a novel influenza A virus. Confirmation as swine influenza A (H1N1) virus is now performed at the CDC but may be available in state public health laboratories soon.(9)
While most persons who have had confirmed or suspected 2009 H1N1 influenza have had a mild, uncomplicated self-limited respiratory illness similar to typical seasonal influenza, and while persons not considered to be at increased risk of developing severe or complicated illness may not require treatment, they can be considered for antiviral treatment. Benefits of treating such patients might include a reduced duration of illness. However, based on experience with seasonal influenza treatment, patients not considered to be at increased risk of developing severe or complicated illness and who have mild, uncomplicated illness are not likely to benefit from treatment if initiated more than 48 hours after illness onset (See Figure 1).(10)
Figure 1
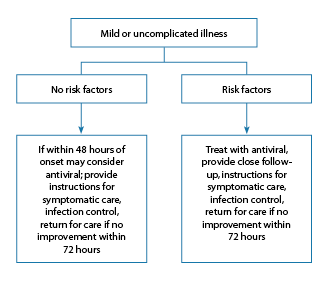
According to the CDC, swine influenza A (H1N1) is susceptible to the neuraminidase inhibitor antiviral medications zanamivir and oseltamivir. It is resistant to amantadine and rimantadine. Treatment recommendations are as follows:(9)
- Suspected cases: Treat with zanamivir alone or with a combination of oseltamivir and either amantadine or rimantadine as soon as possible after the onset of symptoms and for a duration of 5 days.
- Confirmed cases: Zanamivir or oseltamivir should be administered for 5 days.
- Pregnant women: Antiviral medications are in Pregnancy Category C, so they should be used during pregnancy only if the potential benefit outweighs the potential risk to the embryo or fetus.
- Children younger than 1 year: Because infants typically have high rates of morbidity and mortality from influenza, infants with swine influenza A (H1N1) infections may benefit from treatment with oseltamivir.
Recommendations for treatment of H1N1 can be found at www.cdc.gov/h1n1flu/recommendations.htm; vaccination recommendations are available at www.cdc.gov/h1n1flu/vaccination/acip.htm.
We have often heard the saying that “an ounce of prevention is worth a pound of cure,” so what can you do to prepare for and protect against H1N1? Other than getting vaccinated--have you had your flu shot yet?--the best thing you can do is to wash your hands! Before you eat, before you touch your face, and after you shake hands or come home from running errands. If you work in a hospital or other healthcare facility, you need to be especially cautious. Wash your hands BEFORE and AFTER you enter patients’ rooms, and wipe down equipment that is moved from room to room, such as portable monitors. And if you wear a gown to tend to a patient, don’t wear it to the cafeteria! Dispose of it immediately. Flu.gov provides a summary of everyday steps you can do to protect your own health and the heath of others.(11)
- Cover your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
- Wash your hands often with soap and water, especially after you cough or sneeze. Alcohol-based hand cleaners are also effective.
- Avoid touching your eyes, nose, or mouth. Germs spread this way.
- Try to avoid contact with sick people.
- Stay at home if you are sick until at least 24 hours after you no longer have a fever (100°F or 37.8°C) or signs of a fever (without the use of a fever-reducing medicine such as Tylenol).
- Follow public health advice regarding school closures, avoiding crowds, and other social distancing measures.
Being prepared for how to handle flu pandemic that impacts the business environment is also important. A number of resources regarding H1N1 preparedness in the workplace are available from sources such as the CDC, EEOC (Equal Employment Opportunity Commission), and OSHA. CDC guidelines for employers are available at www.cdc.gov/h1n1flu/business/guidance. EEOC offers recommendations for emergency preparedness in the workplace at www.eeoc.gov/facts/pandemic_flu.html. OSHA provides guidance on preparing workplaces for an influenza pandemic at www.osha.gov/Publications/influenza_pandemic.html, in addition to providing a fact sheet on pandemic flu at www.osha.gov/Publications/employers-protect-workers-flu-factsheet.html.
For those of you interested in tracking influenza activity in the United States, visit FluView at www.cdc.gov/flu/weekly, a weekly influenza surveillance report prepared by the CDC. Data is available on a national and regional level, and includes valuable information on virologic surveillance, hospitalization, death tracking, and geographic spread of influenza by state.
As sure as things change, they also stay the same. Since 1918, there is little doubt that our medicine and our lifestyle has advanced, our understanding of disease progression and transmission has improved, but it is still the simplest steps that can help to prevent the spread of disease. As true today as it was almost a hundred years ago, covering your mouth when you sneeze or cough, or sneezing into your elbow or shoulder as is now recommended (http://answers.flu.gov/questions/3764), and washing your hands are still the best things you can do to prevent spread of the flu. After 91 years of research, fundamental questions about influenza pandemics remain unanswered. Thus, we must remain vigilant and use the knowledge we have gained from 1918 and other influenza pandemics to direct targeted research and pandemic influenza preparedness planning, emphasizing prevention, containment, and treatment.(5)

Photo Credit: National Library of Medicine
Do you have feedback for the author? Click here to send us an email.
Download printable version here.
References
- United States Department of Health and Human Services (DHHS). The Great Pandemic – The United States from 1918-1919. Available at http://1918.pandemicflu.gov/the_pandemic/index.htm.
- Heinrich J. FLU VACCINE: Recent supply shortages underscore ongoing challenges. Testimony before the Subcommittee on Health and the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, House of Representatives. United States Government Accountability Office (GAO). 2004. Available at http://www.gao.gov/new.items/d05177t.pdf.
- Dimitrov N, Goll S, Meyers LA, Pourbohloul B, Hupert N. Optimizing Tactics for use of the U.S. Antiviral Strategic National Stockpile for Pandemic (H1N1) Influenza, 2009. PLoS Curr Influenza 2009;Nov 4:RRN1127.
- Centers for Disease Control (CDC). Seasonal Influenza (flu): The Disease. 2009. Available at http://www.cdc.gov/flu/about/disease.
- Taubenberger JK, Harvey HA, Memoli MJ. The 1918 influenza pandemic: Lessons for 2009 and the future. Crit Care Med 2009 Dec 31;[Epub ahead of print].
- Haque N, Bari MS, Bilkis L, Hossain MA, Islam MA, et al. Swine flu: a new emerging disease. Mymensingh Med J 2010;19:144-149.
- Centers for Disease Control (CDC). 2009 H1N1 Flu ("Swine Flu") and You. 2009. Available at http://www.cdc.gov/H1N1flu/qa.htm.
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009;325:197-201.
- Yox SB. Swine Flu: Guidance and Resources for Clinicians. Medscape Infectious Diseases. 2009. Available at http://www.medscape.com/viewarticle/702050.
- Centers for Disease Control (CDC). Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season. 2009. Available at http://www.cdc.gov/h1n1flu/recommendations.htm.
- U.S. Department of Health & Human Services. Prevention and Treatment. 2010. Available at http://www.flu.gov/individualfamily/prevention/index.html.
|
|