 |
|||||||||||||||||||||||||
| Subscribe to Clinical Compass™ | VOLUME 3, ISSUE 2 - JANUARY 15, 2008 | ||||||||||||||||||||||||
 |
|||||||||||||||||||||||||
|
The Adverse Effects of the Black Box Warning on Antidepressants by Mary Ann Stephens, PhD The "black box" required on all antidepressants warning about an association between antidepressant use and increased risk of suicidal ideation and attempts was supposed to protect, not harm, children through improved monitoring of symptoms during the initial weeks of pharmacotherapy. Yet the black box has led to an unintended effect of instilling fear of antidepressant use among patients and prescribing physicians as well, a decrease in antidepressant use, and an unprecedented increase in suicides for that age group in the years following the warning.(1) Last year, the warning was extended to young adults upon further review of clinical trials that included people under 25 years of age. Not surprisingly, many physicians and mental health and suicide prevention organizations are increasingly voicing their concerns about these trends, and continue to object to the placement of the black box on antidepressants. "Studies in children and adolescents have shown that antidepressants can increase suicidal thoughts. However, other studies have shown that the overall risk of attempting suicide goes down after starting antidepressant medication," states Gregory Simon, MD, MPH, from the Center for Health Studies in Seattle. The risk of serious suicide attempt during the first 6 months of antidepressant treatment is less than 1 per 1,000 for adults and less than 1 in 300 for adolescents.(2) "As a physician I was always taught to consider the risk-to-benefit ratio of any treatment and here the risk is very low. In fact, not treating depression carries a far greater risk for suicide than any potential adverse effects of these medications," said Paula Clayton, MD, Medical Director of the American Foundation for Suicide Prevention, speaking at the FDA hearing. What do you think about the black box warning on antidepressants? In an informal poll of neuroscienceCME Clinical Compass subscribers, we asked two questions related to the unintended effect of the black box warning. While the majority of subscribers felt that the warning has had a negative impact on outcomes for children and young adults and that clinicians are being overly cautious, a substantial proportion do not feel this way, and an equally substantial proportion were not sure. 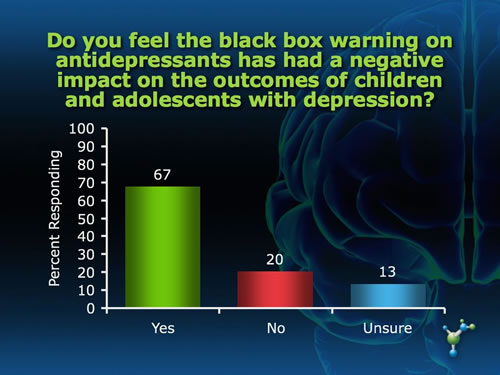
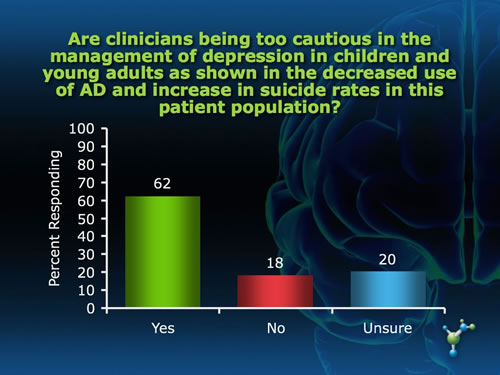
And, what about the data reviewed by the FDA that led to the requirement of the black box warning in the first place? The debate about suicide risk and antidepressant use is clearly more complicated than can be summed up in a black box warning, pointing to the need for physicians to be educated on the issues surrounding the debate. Join CME Outfitters at neuroscienceCME.com on January 30 at 12 p.m. ET to hear the experts discuss this very important topic about antidepressants, the black box warning, and the concerns of untreated depression, and receive valuable guidance on how to address patients' concerns about antidepressants and how to monitor symptoms of depression during practice. For more information, visit http://www.neurosciencecme.com/cmea.asp?ID=272 Do you have feedback for the author? Click here to send us an email. References
|
|||||||||||||||||||||||||
| ©2008 CME Outfitters, LLC |
|||||||||||||||||||||||||